The microbial flora involved in human
The human gastrointestinal tract is home to diverse and vast communities of microorganisms. There are about 10 times as many microbial cells and 100 times as many microbial genes in the human body as there are human cells. The human microbiome includes around 100 trillion bacterial cells and an adult human typically has around 10 trillion human cells. The human microbiome is a community of microorganisms that can be found all over our body surfaces, especially in the mouth, gut and vagina, as well as the skin and eyes. The colonisation of the gastrointestinal tract begins immediately after birth and most of the bacteria inhabit the large intestine. Human microbiome is as unique as a fingerprint. Different people harbor different collections of microorganisms.
The Large-scale study into human microbiome
The NIH Human Microbiome Project (HMP) analysed an impressive 4,788 specimens. The project found enormous variations in microbial populations from person to person. This large-scale, well-funded and widely published research is exciting as it demonstrates the growing medical focus on human bacteria in the gut and the rest of the body. The HMP will not only identify new ways to determine health and predisposition to diseases but also define the parameters needed to design, implement and monitor strategies for intentionally manipulating the human microbiota, to optimize its performance in the context of an individual's physiology.
The concept of a core human microbiome
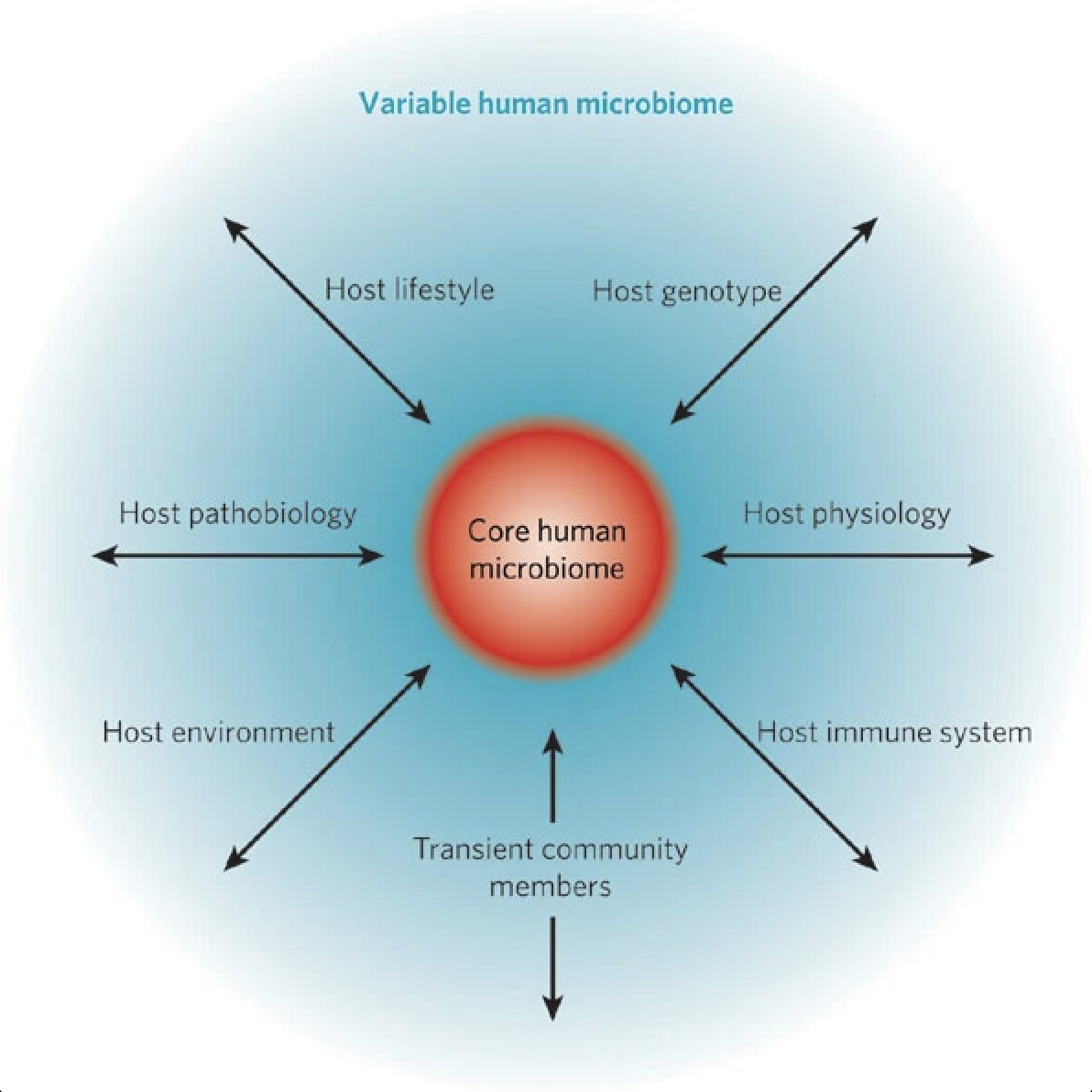
The core human microbiome (red) is the set of genes present in a given habitat in all or the vast majority of humans. Habitat can be defined over a range of scales, from the entire body to a specific surface area, such as the gut or a region within the gut.
The variable human microbiome (blue) is the set of genes present in a given habitat in a smaller subset of humans. This variation could result from a combination of factors such as host genotype, host physiological status (including the properties of the innate and adaptive immune systems), host pathobiology (disease status), host lifestyle (including diet), host environment (at home and/or work) and the presence of transient populations of microorganisms that cannot persistently colonize a habitat.
Gut microbiome is the key for human health
The gut microbiome can be regarded as a metabolically active organ and modulation thereof by probiotics or prebiotics is becoming increasingly recognized as an important therapeutic option1. The gut microbiome is important for overall health as it supports gut2,3 and immune health4,5, metabolism6,7 and may also influence the gut-brain axis8,9,10. So great is the role being identified for the gut microbiota in our health, that it is becoming widely regarded as an ‘organ’ in its own right, an 'organ' which appears to play a key role in immune function. It helps you develop and maintain a balanced immune system11,12 and helps support integrity of the intestinal barrier13,14.
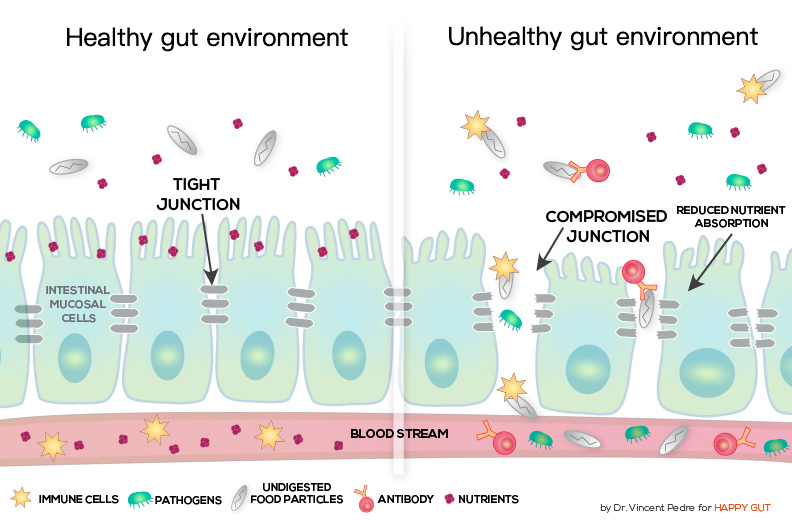
As the mucosal tissues of the gut wall comprise some of the most important barriers involved in innate immunity, these areas can significantly influence the integrity of our immune function1. At least 70% of our immune cells are located in the gut, housed in structures buried in the delicate hair-like villi which cover the intestinal wall. In a healthy individual (healthy gut environment), gut bacteria stimulates the immune system, which essentially talks back in return. However an imbalance in the gut microbiota caused by modern lifestyles, overuse of antibiotics and poor diet can disrupt the conversation between these cells leading to a break down in communication15. If the integrity of the gut wall is compromised, this can lead to unhealthy gut environment and allow larger protein molecules to pass through the intestinal wall and into the bloodstream, where they are seen as unnatural ‘predators’ that can stimulate inappropriate immune responses that we know as an ‘allergy’.
Probiotics may help to mediate allergy symptoms in a number of ways. They may help to improve the integrity of the intestinal lining, preventing permeability of the tight junctions in the epithelial wall. Probiotics are thought to help to alleviate and prevent this situation by displacing the pathogens that originally cause the inflammation and by helping to promote the secretion of anti-inflammatory mediators involved in the healing process.
- 1. Montalban-Arques A., et al. (2015). Selective Manipulation of the Gut Microbiota Improves Immune Status in Vertebrates. Frontiers in Immunology. Oct 9;6:512..
- 2. Dimidi E., et al. (2017). Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr. 8(3):484-494.
- 3. Kho Z.Y., Lal S.K. (2018). The Human Gut Microbiome - A Potential Controller of Wellness and Disease. Front Microbiol. 9:1835.
- 4. Arumugam M., et al. (2011). Enterotypes of the human gut microbiom. Nature. 473(7346):174-180.
- 5. Araos R., D’Agata E.M.C. (2019). The human microbiota and infection prevention. Infect Control Hosp Epidemiol. 40(5):585-589.
- 6. Ortega M.A., et al. (2020). Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients. 12(9):1-29.
- 7. Tseng C.H., Wu C.Y. (2019). The gut microbiome in obesity. J Formos Med Assoc. 118:S3-S9.
- 8. Osadchiy V., et al. (2019). The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol. 17(2):322-332.
- 9. Bastiaanssen T.F.S., et al. (2019).Making Sense of … the Microbiome in Psychiatry. Int J Neuropsychopharmacol. 22(1):37-52.
- 10.Warner B.B. (2019). The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr Res. 85(2):216-224.
- 11.Jeyakumar T., et al. (2019). Impact of the Microbiome on the Human Genome. Trends Parasitol. 35(10):809-821.
- 12.Lambring C.B.,et al. (2019). Impact of the Microbiome on the Immune System. Crit Rev Immunol. 39(5):313-328.
- 13.Kho Z.Y., Lal S.K. (2018). The Human Gut Microbiome - A Potential Controller of Wellness and Disease. Front Microbiol. 9:1835.
- 14.Paone P., Cani P.D. (2020). Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 69(12):2232-2243.
- 15.Chen‐Yu Hsieh et al, (2015) Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum, Physiological Reports Vol. 3 no. e12327
